Mechanism for the formation of a cyanohydrin:
The intermediate formed can now work like a nucleophile or base since it is negatively charged and it reacts with the acidic hydrogen of HCN. A lone pair of electrons from oxygen is employed to make a bond to the acidic proton and the H-CN σ bond is broken at similar time like that these electrons move onto the neighboring carbon to give it a lone pair of electrons and a negative charge (Step 2). The products are the cyanohydrin and the cyanide ion. Note: A cyanide ion started the reaction and a cyanide ion is again generated.
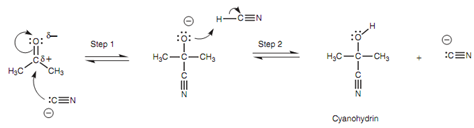
Figure: Mechanism for the formation of a cyanohydrin.
Hence, only a catalytic amount of cyanide ion is needed to start the reaction and once the reaction has taken place, a cyanide ion is again generated to carry on the reaction with other molecule of ketone.
Cyanohydrins are helpful in synthesis since the cyanide group can be transformed to an amine or to a carboxylic acid.
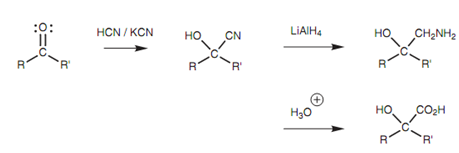
Figure: Further reactions of cyanohydrins.