Bisulfite addition:
The reaction of an aldehyde or a methyl ketone along with sodium bisulfite (NaHSO3) includes nucleophilic addition of a bisulfite ion (-:SO3H) to the carbonyl group to provide a water soluble salt. The bisulfite ion is a comparatively weak nucleophile as compared to other charged nucleophiles and thus only the most reactive carbonyl compounds will react. Larger ketones do not react because larger alkyl groups hinder attack. The reaction is as well reversible and thus it is a helpful method of separating aldehydes and methyl ketones from other ketones or from other organic molecules. This is generally done during an experimental work up in which the products of the reaction are dissolved in a water immiscible organic solvent. After that aqueous sodium bisulfite is added and the mixture is shaken carefully in a separating funnel. One time the layers have separated, any aldehydes and methyl ketones will have hone through nucleophilic addition with the bisulfite solution and will be dissolved in the aqueous layer like the water soluble salt.
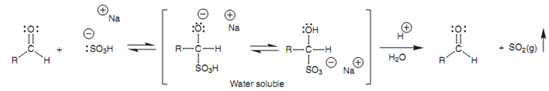
Figure: Reaction of the bisulfite ion with an aldehyde.
Now the layers can be separated. If the aldehyde or methyl ketone is needed, it can be recovered through adding acid or base to the aqueous layer that reverses the reaction and again generates the carbonyl compound.