Sigma bonds:
A half-?lled sp3 hybridized orbital from one carbon atom can be employed to make a bond with a half-?lled sp3 hybridized orbital from other carbon atom. In diagram, the main lobes of the two sp3 orbitals overlap straight leading to a strong σ (Sigma) bond. It is the capability of hybridized orbitals to make strong σ (Sigma) bonds that describes why hybridization occurs in the ?rst place. The deformed dumbbell shapes permit a much better orbital overlap than would be acquired from a pure s orbital or a pure p orbital. A σ (Sigma) bond among an sp3 hybridized carbon atom and a hydrogen atom includes the carbon atom by using one of its half-?lled sp3 orbitals and the hydrogen atom by using its half-?lled 1s orbital.
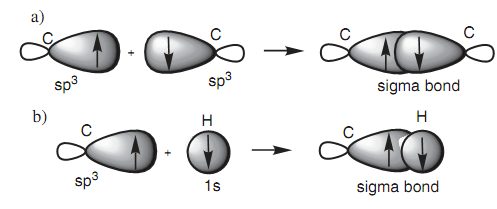
Diagram: (a) σ Bond between two sp3 hybridized carbons; (b) σ bond between a sp3 hybridized carbon and hydrogen