Geometry of hybridized nitrogen:
Alcohols, amines, alkyl halides, and ethers all consists of sigma bonds including nitrogen, oxygen, or chlorine. Bonds among these atoms and carbon are created through the overlap of half- filled sp3 hybridized orbitals from every atom. Bonds including hydrogen atoms (for example O-H and N-H) are created by the overlap of the half- filled 1s orbital from hydrogen and a half- filled sp3 orbital from oxygen or nitrogen.
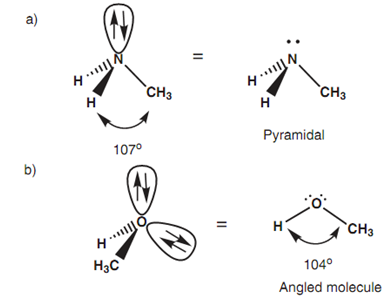
Figure: (a) Geometry of sp3 hybridized nitrogen; (b) geometry of sp3 hybridized oxygen.