Geometry:
Each of the sp3 hybridized orbitals has similar shape - a rather deformed looking dumbbell. This deformed dumbbell looks much more like a p orbital than an s orbital because more p orbitals were included in the mixing process.
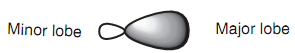
Figure: sp3 Hybridized orbital.
Each sp3 orbital will take place a space as far apart from each other as possible through pointing to the corners of a tetrahedron. At this time, only the main lobe of each hybridized orbital has been displayed and the angle among each of these lobes is 109.5o. This is what is meant through the expression tetrahedral carbon. The three- dimensional (3D) shape of the tetrahedral carbon can be presented by drawing a normal line for bonds in the plane of the page. Bonds going behind the page are presented through a hatched wedge, and bonds coming out the page are presented through a solid wedge.
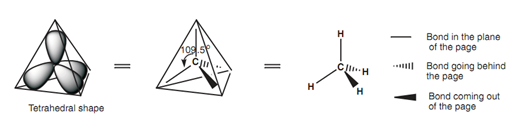
Figure: Tetrahedral shape of a sp3 hybridized carbon