Electronic configuration:
For carbon the valence electrons can now be fitted into the sp3 hybridized orbitals. There were a total of 4 electrons in the original 2s and 2p orbitals. The s orbital was full filled and two of the p orbitals were half filled. Later than hybridization, there is a total of four hybridized sp3 orbitals all of equivalent energy. By Hund's rule, they are all half ?lled with electrons that means that there are 4 unpaired electrons. Now Four bonds are possible.
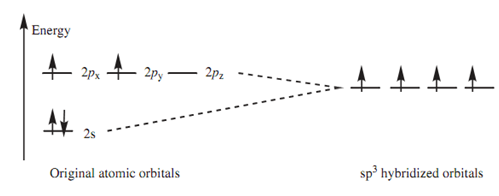
Figure: sp3 Hybridization.