Geometry:
The 2py orbital has the general dumbbell shape. Each one of the sp2 hybridized orbitals comprises a deformed dumbbell shape identical to a sp3 hybridized orbital. Though, the variation among the sizes of the major and minor lobes is larger for the sp2 hybridized orbital. The hybridized orbitals and the 2py orbital occupy spaces thus far separate from each other as possible. The lobes of the 2py orbital comprise the space above and below the plane of the x and z axes. The three sp2 orbitals (major lobes displayed only) will then occupy the remaining space like that they are as far separate from the 2py orbital and from each other as possible. The result of it is that, they are all located in the x-z plane pointing in the direction of the corner of a triangle (trigonal planar shape). The angle among each of these lobes is 120°. We are now ready to look at the bonding of a sp2 hybridized carbon.
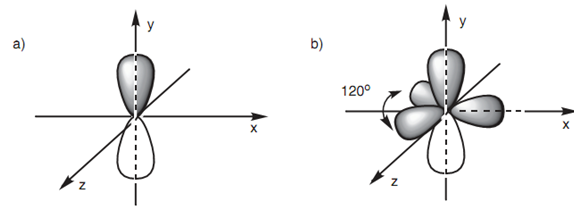
Figure: (a) Geometry of the 2py orbital; (b) geometry of the 2 py orbital and the sp2 hybridized orbitals.