Conjugated systems:
Aromatic rings are not the just only structures in which delocalization of π electrons can occur. Delocalization takes place in conjugated systems in which there are alternating single and double bonds (for example 1, 3-butadiene). All 4 carbons in 1,3-butadiene are sp2 hybridized and thus each of these carbons comprise a half-filled p orbital that can interact to provide two π bonds. Though, a specific amount of overlap is as well possible among the p orbitals of the middle two carbon atoms and thus the bond that are connecting the two alkenes has a number of double bond character - borne out through the observation that this bond is shorter in length as compared to a typical single bond. This delocalization as well results in increased stability. Though, it is significant to realize that the conjugation in a conjugated alkene is not as great like in the aromatic system. Within the latter system, the π electrons are totally delocalized round the ring and all the bonds are equivalent in length. In 1, 3-butadiene, the π electrons are not completely delocalized and are more possible to be found in the terminal C-C bonds. Even though there is a specific amount of π character in the middle bond, the latter is more similar to a single bond as compared to a double bond. Other instances of conjugated systems involve α, β-unsaturated ketones and α, β- unsaturated esters. These also have increased stability because of conjugation.
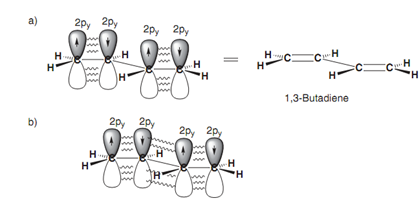
Figure: (a) π Bonding in 1, 3-butadiene; (b) delocalization in 1,3-butadiene
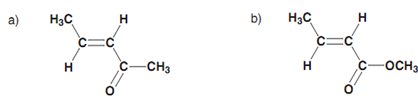
Figure: (a) α, β-Unsaturated ketone; (b) α, β-unsaturated ester.