Aromatic rings:
Each carbon in an aromatic ring is sp2 hybridized that means that each carbon can make three σ bonds and one π bond. In diagram, all the single bonds are σ whereas each double bond contains one σ bond and one π bond. Though, this is an oversimplification of the aromatic ring. For instance, double bonds are shorter as compared to the single bonds and if benzene had this specific structure, the ring would be deformed with longer single bonds as compared to the double bonds.
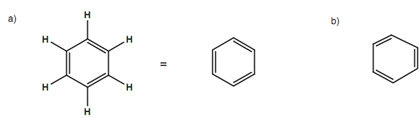
Figure: (a) Representation of the aromatic ring; (b) 'deformed' structure resulting from fixed bonds.