Alkenes:
Sp2 Hybridization effects in three half-filled sp2 hybridized orbitals that form a trigonal planar shape. The make use of these three orbitals in bonding describes the shape of an alkene, for instance ethene (H2C = CH2). As far as the C-H bonds are concerned, the hydrogen atom employs a half-filled 1s orbital to make a strong σ bond along with a half filled sp2 orbital from carbon. A strong σ bond is as well possible among the two carbon atoms of ethene because of the overlap of sp2 hybridized orbitals from each carbon.
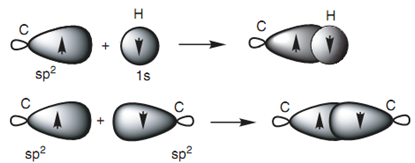
Figure: (a) Formation of a C-H σ bond; (b) formation of a C-C σ bond.
The full σ bonding diagram for ethene is displayed in diagram and can be simplified as displayed in the below diagram. Ethene is a flat, rigid molecule in which each carbon is trigonal planar. We have described how sp2 hybridization describes the trigonal planar carbons but we have not described why the molecule is rigid and planar. If the σ bonds were the just only bonds available in ethene, the molecule would not remain planar because rotation could take place round the C-C σ bond. Hence, there must be additional bonding which 'locks' the alkene into this planar shape.