High-energy compounds
The ability to produce high-energy compounds for metabolism and storage is a prerequisite for cell survival. Energy is acquired by cells through a series of balanced oxidation- reduction (redox) reactions from organic or inorganic substrates. The simplest redox reaction can be seen in the reaction below.
H2 + ½ O2 --> H2O
H2 = reductant (electron donor) that becomes oxidized
O2 = oxidant (electron acceptor) that becomes reduced
The energy which is released in redox reactions is stored in a variety of organic molecules which contain oxygen atoms and phosphate groups. ATP adenosine triphosphate is a high-energy compound found in almost all living organisms. It is synthesized in catabolic reactions where substrates are oxidized and utilized in anabolic biosynthetic reactions. Intermediates called carriers participate in the flow of energy from the electron donor to the terminal electron acceptor. The co-enzyme nicotinamide adenine dinucleotide (NAD+) is a freely diffusible carrier that transfers two electrons plus a proton and a second proton from water to the next carrier in the chain.
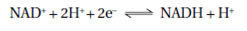
The reactions for the phosphorylated derivative (NADP+) are same. NAD+ is usually used in energy-generating reactions and NADP+ in biosynthetic reactions.
All protozoa, fungi and most prokaryotes synthesize ATP by oxidizing organic molecules. This can be either via respiration or by fermentation. Respiration requires a terminal electron acceptor. This is usually oxygen but nitrate and sulfate are among the compounds used in anoxic conditions. Fermentation requires an organic terminal oxygen acceptor.
Microorganisms can be collected according to the source of energy they use and by the source of carbon which may either be an organic molecule or from CO2 carbon dioxide fixation in Table 1.
