Hardening
If a steel part is heated 30-50oC above A3 temperature complete austenising is permitted by soaking at that temperature and then cooled suddenly (quenched), the breakdown of austenite is suppressed. The new phase that composes is martensite in which all the dissolved carbon is held in form of body centered tetragonal structure shown in Figure. Martensite is only metastable phase and might be tempering. It is extremely hard and brittle and has a characteristic acicular appearance when examined under microscope under high magnification. In the steels up to eutectoid composition, the martensite formed by this drastic quenching operation contains all the carbon that was contained in austenite. In higher eutectoid steels, however, some carbon is converted into carbide particles also.
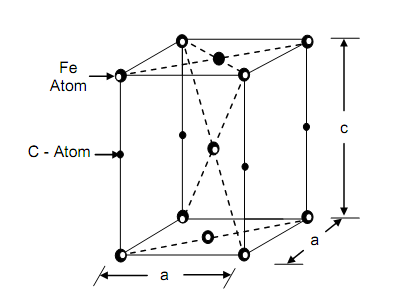
Figure: The Unit Cell of Distorted Body-centered Tetragonal Lattice of Martensite
The hardness of martensite is dependent upon the percentage of carbon present in structure. Figure illustrates how this hardness varies with carbon content. It can be seen that hardness of plain carbon steels increases rapidly until the eutectoid composition is reached. After this composition the hardness increases very normally. The fact is that the hardest martensite is formed at eutectoid composition while hardness remains at this level in hypereutectoid steels. The slight increase in hardness of hypereutectoid steel is due to formation of carbide particles which are hard and brittle.
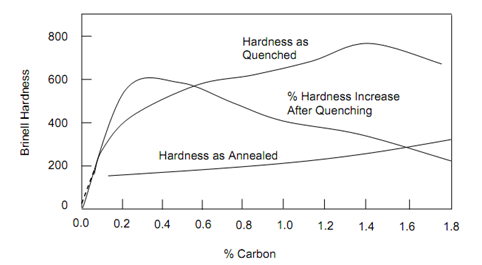
Figure: Variation of Hardness of Martensite with Carbon Content of Steel