Oxides and Oxoacids
I2O5 is the just halogen oxide of moderate thermodynamic stability. Other compounds comprise X2O (not I), X2O2 (F and Cl), the odd-electron XO2 (Cl and Br), and Cl2O7. Several of these compounds are strongly oxidizing, contain low thermal stability and can decompose explosively. ClO2 is used like a bleaching agent.
Apart from fluorine the elements have an extensive oxoacid chemistry Figure 1 depicts Frost diagrams by the oxidation states found in acid and alkaline solution. The sharp tendency in oxidizing power of the elements (X2/X- potential) can be seen. As supposed from Pauling's rules the hypohalous acids X(OH) and chlorous acid ClO(OH) are weak acids, but the halic acids XO2(OH) and particularly perchloric acid ClO3(OH) and perbromic acid are strong. Periodic acid is exceptional, as, even though periodates consisting of the tetrahedral IO-4 ion are well known, the predominant form in water is the octahedral IO(OH)5, that, as supposed, is a weak acid.
The redox behaviour is strongly dependent on pH but is also affected through kinetic factors. From the pH=14 diagram in diagram 1 it can be observe that Cl2 and Br2 disproportionate in alkaline solution. The thermodynamically supposed results
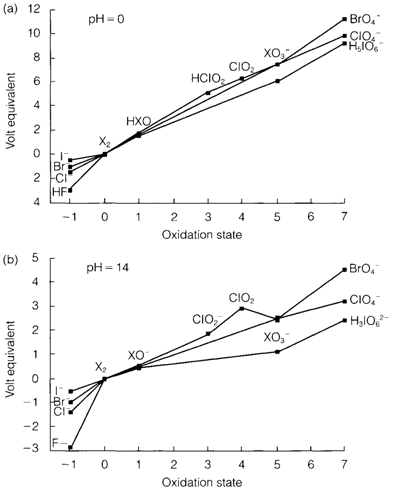
Fig. 1. Frost diagrams for the halogens in aqueous solution at pH=0 (a) and pH=14 (b). X represents any halogen, apart from F for positive oxidation states.
Are X- and XO-3but the hypochlorite ion ClO- is created in cold conditions and further disproportionation takes place on heating?
The perhalic acids and their anions are strong oxidizing agents, particularly BrO4- that is not thermodynamically stable in aqueous solution. Though, they do have significant kinetic stability. Perchlorates of organometallic or organic cations are extremely dangerous like they may appear stable but can explode unpredictably with great force.