Iodoform reaction:
α-Halogenation can as well be performed in the existence of base. The reaction carries on by an enolate ion that is after that halogenated. Though, it is difficult to stop the reaction at mono-halogenation because the resulting product is usually more acidic than the starting ketone because of the electron-withdrawing effect of the halogen. The result of it is that another enolate ion is rapidly formed leading to further halogenation.
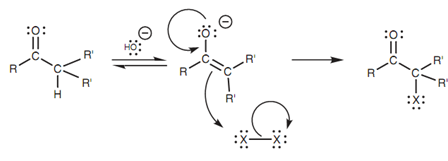
Figure: α-Halogenation in the presence of base.
This trend towards multiple halogenations is the basis for a classical test termed as the iodoform test that is employed to identify methyl ketones. The ketone to be tested is treated along with excess iodine and base and if a yellow precipitate is made, a positive result is pointed out. Within these conditions, methyl ketones go through α-halogenation three times. The product acquired is then susceptible to nucleophilic substitution whereby the hydroxide ion substitutes the tri-iodomethyl (-CI3) carbanion - a good leaving group due to the three electron-withdrawing iodine atoms. Tri-iodomethane is then formed as the yellow precipitate.
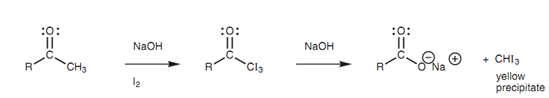
Figure: The iodoform reaction.