MIII compounds
All aluminum halides can be made through direct reaction, but AlF3 is best created through reaction with anhydrous HF. It has a structure that is based on corner-sharing AlF6 octahedra (identical to ReO3; see Topic D3). Solid AlCl3 has a polymeric layer structure, but within the gas phase or nonpolar solvents is dimeric and molecular Al2Cl6.
The iodide and bromine comprise the molecular dimeric form in the solid state. The Aluminum halides are very strong Lewis acids and AlCl3 is often used as an acid catalyst, for instance, in organic Friedel-Crafts reactions.
Complex halides consisting of the ions [AlF6]3- and [AlCl4]- are simply created and can be helpful for the preparation of compounds consisting of unusual cations like Cd22+.
The very stable form of the oxide Al2O3 is α-alumina with the corundum structure in which Al3+ ions take place 2/3 of the octahedral holes in a hexagonal close-packed oxide lattice. Other form γ-Al2O3 has a defect spinel structure. So-called β-alumina is actually a mixed oxide of aluminum of estimated formula NaAl11O17 with a disordered arrangement of Na+ ions and is a good ionic conductor.
Aluminum creates several mixed oxides of which the aluminosilicates are main constituents of minerals. Aluminum occasionally replaces a portion of the silicon exists as corner-sharing SiO4 groups in these compounds. The mixed oxide mineral spinel MgAl2O4 provides its name to a significant structure type. 1/2 of the octahedral holes and one-eighth of the tetrahedral holes are filled within a cubic close-packed array of oxide ions. In the normal spinel form that is adopted through MgAl2O4 the divalent Mg2+ ion is in tetrahedral sites and the trivalent Al3+ is octahedral. A fraction of the cation sites are occupied at random in the defect spinel structure of γ-Al2O3.
Oxides and Halides of Ga and In are quite identical to those of Al, but contain less negative enthalpies of formation and (with In) a trend to higher coordination. TlIII is more strongly oxidizing; for instance, there is no TlIII iodide and the compound of stoichiometry TlI3 actually consists of Tl1 with the linear tri-iodide ion I3 -.
Al, Ga and In create tetrahedrally coordinated solids with elements of group 15, that are part of the series of III-V semiconductors (that is groups 13-15, III-V in old nomenclature). The varied compounds gallium aluminum phosphide Ga1-xAlxP and the arsenide Ga1-xAlxAs are employed for light-emitting diode (LED) shows and semiconductor lasers.
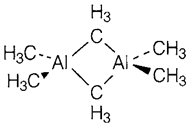
Aluminum hydrides AlH3 comprise a structure identical to that of AlF3. The tetra-hydroaluminate ion [AlH4]- is a very powerful reducing and hydride transfer agent, usually used in the form of lithium aluminum hydride' LiAlH4 made through reaction of LiH with AlCl3. Hydrides' stability decreases down the group but [GaH4]- is quite stable and the unstable digallane molecule Ga2H6 has been recognized with a structure such as that of diborane. Organoaluminum compounds are dimeric but the bonding is dissimilar from that of halides like the bridging methyl groups in Al2(CH3)6 (1) must be held through three-center two-electron bonds identical to those in diborane. Organometallic compounds of Ga, In and Tl than for Al and do not dimerize are less stable.