MIII aqueous chemistry
Al3+ is amphoteric and will dissolve in alkaline and acidic solutions. The [Al(H2O)6]3+ ion is created at low pH but goes through increasing protolysis as the pH rises above four, and polymeric species like [Al13O4(OH)24(H2O)12]7+ can be identified. The extremely insoluble Al(OH)3 is created at neutral pH but redissolves above pH 10:
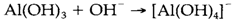
Al3+ depicts typically 'hard' complexing behavior and has a especially strong affinity for negative charged and/or chelating ligands, like (C2O42-)oxalate and EDTA. Ga3+ is identical to Al3+ but In3+ is more basic. Tl3+ is different as it is a strong oxidizing agent, readily creating Tl+ (see below and Fig. 1). It also depicts strong class b or 'soft' complexing behavior, even though not so marked like that of Hg2+.