Graphite furnace:
A graphite furnace consists of a hollow graphite cylinder having a length of about 5 cm and diameter of about 1 cm. A tube is surrounded through a metal jacket by that water is circulated and remains separated from the tube through a gas space where as an inert gas such as argon or nitrogen is circulated as schematically display in Figure. A little amount of analyte sample solution (1-100 µ L) is introduced within the sample cell or holder through inserting the tip of micropipette by a port in the outer jacket, and within the gas inlet orifice in the middle of the graphite tube. On the other hand the powered analyte sample (about 10-500 µg) is introduced directly within the graphite tube.
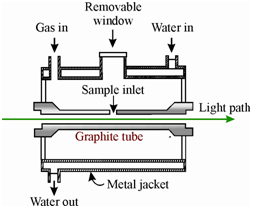
Figure: Schematic diagram of the cross section of an electrically heated graphite furnace
The nature and design of cuvettes or sample holder is of great significance in GFAAS. Various types of cuvettes are commercially available. The standard cuvette made from electrographite is appropriate for the determination of volatile elements like as Pb and Cd. Extended lifetime cuvettes could sustain faster heating rate and have longer lifetime and are especially meaningful in the determination of refractory elements.
The graphite tube is heated through the passage of an electric current to a temperature capable of evapourating the solvent from the solution. A current is then increased within such a way in which initially the sample is ashed and then ultimately it is vaporised producing metal atoms. Instead, a heating cycle as displays in Figure (a) is followed. For reproducibility, the temperatures and the time of the ashing, drying, and atomisation procedure are carefully selected depending on the metal to be analyzed.