Weathering and Sedimentation
Sedimentary processes start with weathering of rocks, a chemical breakdown generated through the action of water and atmospheric CO2. A general reaction is the weathering of potassium feldspar (KAlSi3O8) to create the clay mineral kaolinite:
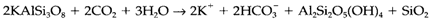
CO2 works to give acid in this reaction, and weathering is accelerated through living organisms that give CO2 by respiration and decay. An additional step in this procedure leads to extremely insoluble Al(OH)3:
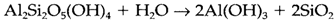
So rocks are transformed through weathering, with soluble ions like K+ being washed out and insoluble resistates remaining. Several significant sources of elements are of this form, that involving bauxite Al(OH)3, rutile TiO2 and cassiterite SnO2.
The action of atmospheric oxygen on soluble ions might produce insoluble oxidates like Fe(OH)3 and MnO2 from Fe2+ and Mn2+, correspondingly. Another elements pass into the ocean and become deposited in several ways: as biogenic deposits like CaCO3 and SiO2, that originate like the shells of marine organisms, or as evaporates like NaCl produced through evaporation of salt lakes.