Element Classification
Geochemistry is the study of chemistry within the natural environment of the Earth. Most elements presented to us come from the solid rocks of the Earth's crust. Fundamental the crust is the mantle of rather identical composition to the crust, inside that is a metallic core. Overlying the crust are atmosphere and the aqueous environment or hydrosphere of the rivers, oceans, and lakes.
The chemical processes occuring in the crust are particularly significant as they have created ores, concentrated mineral deposits which are exploited industrially like sources of particular elements and their compounds. Diagram 1 summarizes the principal chemical forms where each element occurs. At least half the elements take place in the crust like oxides (mostly complex ones like silicates) or less generally like halides, and are called lithophiles. In this class all the highly electropositive metals are. Chalcophiles are elements available in sulfide minerals; these involve some elements chemically identical to sulfur (Se, As) jointly with less electropositive metals of the later transition and posttransition metal groups . Some metallic elements of low reactivity are observed in native (uncombined) form on Earth. They are identified as siderophiles and are common in the Earth's metallic core. A few nonmetallic elements (N, noble gases) take place in uncombined form. like Fig. 1 depicts, a few elements have intermediate behavior and fall in more than one class. For instance, iron is found in both lithophilic (Fe silicates, Fe2O3, etc.) and chalcophilic (FeS2) states.
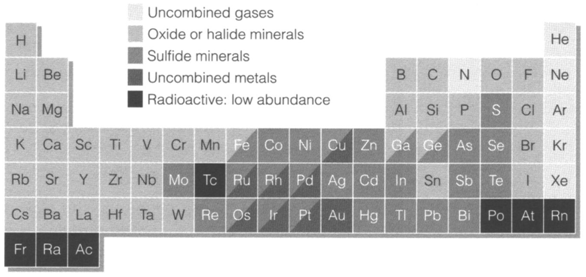
Fig. 1. The periodic table showing the principal types of chemical compound occurring for elements at the Earth's surface. Oxides include many complex forms, especially silicates.