Corrosion of Iron:
Unless remembered otherwise, the following discussion applies to deaerated water at room temperature and around neutral pH. The affects of temperature, oxygen, and pH are discussed later within this theory.
The oxidation and reduction half-reactions inside the corrosion of iron are as given below.
Fe → Fe+2 +2e- (oxidation) (2-3)
H3O+ +e- → H+ H2O (reduction) (2-4)
The whole reaction is the sum of those half-reactions.
Fe +2H3O+ → Fe+2 +2H +2H2O (2-8)
The Fe+2 ions readily combine along with OH- ions at the metal surface, first forming Fe(OH)2 , that decomposes to FeO.
Fe+2 +2OH- → Fe (OH) 2 → FeO +H2O (2-9)
Ferrous oxide (FeO) after that forms a layer on the surface of the metal. Given about 1000(F, therefore, FeO is unstable and undergoes additional oxidation.
2FeO +H2O → Fe2O3 + 2H (2-10)
Atomic hydrogen then reacts to form molecular hydrogen, as elaborates previously, and a layer of ferric oxide (Fe2O3) builds up on the FeO layer. Among these two layers is another layer which has the apparent composition Fe3O4. It is believed in which Fe3O4 is a distinct crystalline state composed of O-2, Fe+2, and Fe+3 in proportions so in which the apparent composition is Fe3O4. These three layers are described in below figure.
At once the oxide film starts to form then the metal surface is no longer in direct contact along with the aqueous environment. For additional corrosion to occur, the reactants must diffuse by the oxide barrier. It is believed in which the oxidation step, the above given Equation (2-3), occurs at the metal-oxide interface. The Fe+2 ions and electrons then diffuse by the oxide layer toward the oxide-water interface. Finally, Fe+2 ions encounter OH- ions and form FeO. The electrons participate within the reduction reaction with hydronium ions. Those latter reactions are believed to take place predominately at the oxide-water interface, other than a few reaction might occur inside the oxide layer through the diffusion of H+, OH-, and H2O within the layer.
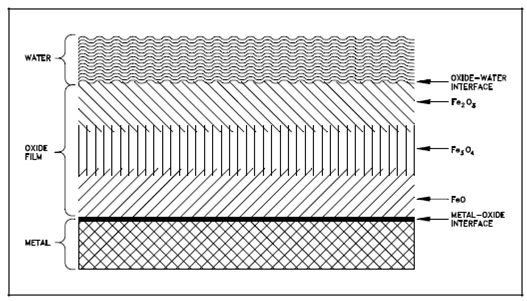
Figure: Simplified Schematic Diagram of Oxide Corrosion Film on the Surface of a Metal
Regardless of the exact diffusion mechanism, an oxide layer represents a barrier to continued corrosion and tends to slow the corrosion rate. The accurate effect of this layer on the corrosion rate depends on the uniformity and tenacity of the film. The metal surface is again exposed to the environment and corrosion occurs more readily if the film is loosely attached, develops defects, or is erased.