Galvanic Cell Showing Absorbed Hydrogen:
The hydrogen atoms created through the reaction of Equation (2-4) absorb on the surface of the metal and remain there until erased through one of two processes: combination of two hydrogen atoms to form molecular hydrogen that is then released as a gas or reaction along with dissolved oxygen to form water. Within the absence of oxygen (deaerated solutions), a first procedure applies.
2H → H2 (2-6)
Combining Equation (2-6) along with Equation (2-4), a net reduction half-reaction is acquired.
2(H3O+ + e- → H+ H2O)
2H → H2 (2-6)
2H3O+ + 2e- → H2 +2H2O (2-7)
Until the absorbed hydrogen atoms are erased from the metal surface, which efficiently blocks the sites at that the reaction of Equation (2-4) could occur. At low temperatures the reaction of Equation (2-6) is slow associative to the reaction of Equation (2-4) since, while the reaction is energetically favored, a combination of two hydrogen atoms needs large activation energy. Equation (2-6) displays the rate-controlling step of the net reduction half-reaction. Since the oxidation half-reaction could occur no faster than the reduction half-reaction, a rate of the whole oxidation-reduction reaction is controlled through the reaction of Equation (2-6).
The layer of absorbed atomic hydrogen is said to polarize the cell. This category of polarization is known as activation polarization and is sometimes referred to while hydrogen polarization or cathodic polarization, since the polarizing reaction occurs at the cathode.
Both concentration and activation polarization reduces the net oxidation-reduction reaction rate. Within corrosion processes, activation polarization commonly has the greater effect.
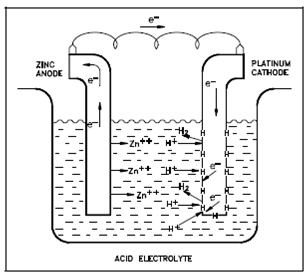
Figure: A Galvanic Cell Showing Absorbed Hydrogen Atoms on a Cathode