Activation of adenylyl cyclase
The Adenylyl cyclase is activated by a particular family of G proteins, the GS proteins, therefore called since their action on adenylyl cyclase is stimulatory. The enzyme catalyzes the alteration of ATP to (cAMP) cyclic adenosine-3’, 5’-monophosphate. The second messenger molecule diffuses freely via the cytoplasm and binds to a kinase enzyme, protein kinase A (PKA) that is thereby switched on as shown in figure. The kinase then phosphorylates aim proteins which have the suitable amino acid series to recognize the kinase.
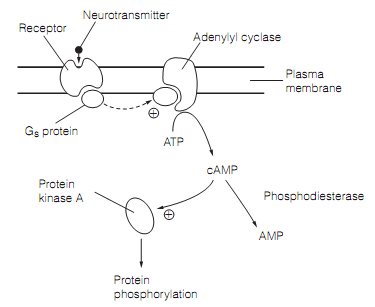
Figure: The adenylyl cyclase-cAMP second messenger system. The activated Gs protein uncouple from receptor to switch on the adenylyl cyclase.
The targets involve ion channels (that is the phosphorylation state of a channel frequently determines whether it is open or closed), and the transcription factors—permitting the cAMP second messenger system to alter gene expression.
A single activated PKA molecule is capable to phosphorylate most of the target proteins, adding to the amplification. The Second messenger cascades should quickly turn off if their signals are to be modulated over a time course of tens or hundreds of milliseconds. For the cAMP system this happens in three ways:
- The Cyclic AMP is hydrolyzed to AMP by the action of a particular phosphodiesterase in the cytoplasm.
- There are particular phosphatases responsible for the dephosphorylating the target proteins. Therefore the phosphorylation state of a protein at a certain time will depend on the balance of the activities of phosphatases and kinases.
- The Prolonged occupation of the receptor by the transmitter causes it to desensitize. This includes phosphorylation by a particular kinase which recognizes the agonist-bound form of the receptor followed by the binding of an arrestin protein. The resultant complex is unable to identify the G protein.