Properties and Reactions:
The organic compounds' chemical and physical properties are ascertained by the sort of intermolecular bonding forces exist that in turn depends upon the functional group present. A molecule like ethane has a low boiling point and is a gas at room temperature since its molecules are bound together via weak van der Waals forces. In difference, methanol is a liquid at room temperature because hydrogen bonding is possible among the alcoholic functional groups.
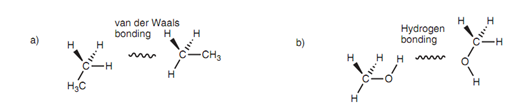
Figure: (a) Intermolecular van der Waals (methane); (b) intermolecular hydrogen bonding (methanol).