Mechanism of carbocation formation:
The Lewis acid (AlCl3) promotes the formation of the carbocation needed for the reaction and does so through accepting a lone pair of electrons from chlorine to create an unstable intermediate that fragments to provide a carbocation and AlCl4.
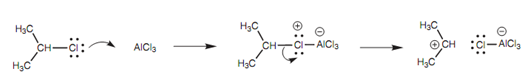
Figure: Mechanism of carbocation formation
One time the carbocation is created it reacts like an electrophile with the aromatic ring through the electrophilic substitution mechanism already explained.
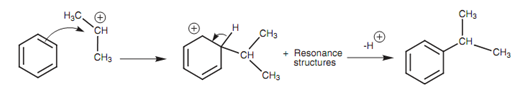
Figure: Mechanism for the Friedel-Crafts alkylation.
There are some restrictions to the Friedel-Crafts alkylation. For instance, the reaction of 1-chlorobutane with benzene provides two products with just only 34% of the wanted product. This is because of the fact that the primary carbocation that is produced can rearrange to a more stable secondary carbocation in which a hydrogen (and the two sigma electrons making up the C-H bond) 'shift' across to the neighboring carbon atom. This is termed as a hydride shift and it occurs because the secondary carbocation is much more stable than the primary carbocation. Such types of rearrangements limit the type of alkylations that can be performed through the Friedel-Crafts reaction.
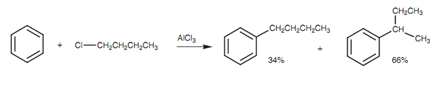
Figure: Friedel-Crafts reaction of 1-chlorobutane with benzene