Jablonski Diagram:
The Jablonski diagram provides a representation of ground and different excited electronic states of a molecule and the processes related along with absorption and emission (radiative and nonradiative) of energy. A classical Jablonski diagram is given in Figure.
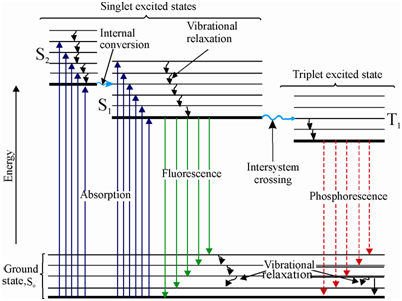
Figure: The Jablonski diagram showing the phenomena of fluorescence and phosphorescence
The set of lines at the bottom of the figure represents the ground state and the ones in the upper portion imply the excited electronic states. To start with, the molecule is in the electronic ground state. In that state, the molecular orbitals are occupied through two electrons. You would recall from your previously knowledge that according to Pauli's principle, the spins of the two electrons in the similar orbital must be antiparallel. This implies in which the total spin, S, of the molecule in the ground state is zero [½ + ( - ½)]. This energy state is known as "singlet state" and is labeled as S0. The electron spins in the excited state achieved through absorption of radiation might either be parallel or antiparallel. Accordingly, this might be a triplet (parallel) or a singlet (antiparallel) state.