Fluorescence with Inorganic Reagents:
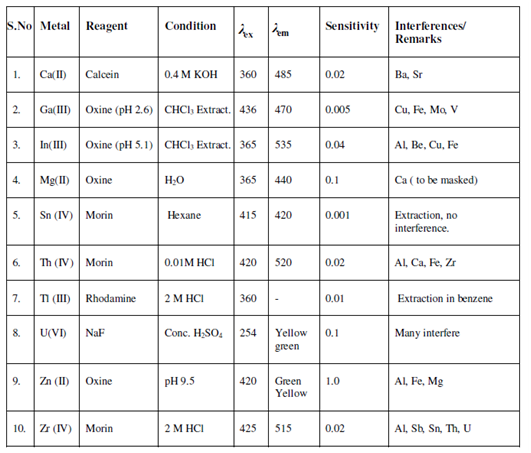
Most of the metals display fluorescence within the presence of appropriate reagents. Instead, these could be analysed through the indirect method. For instance, certain metals like, Tl, Sn, Pb, As, Sb, Bi, can show fluorescence at low temperature on reaction with acids like, HCl, HBr. Most of the p- Block elements in presence of HCl/HBr show fluorescence with excitation wavelength in ultraviolet and emission wavelength in the visible region. For instance, arsenic has λ ex. = 350 nm and λ em. = 690 nm. The detection limit varies from 0.002-1.0 ppm. To one side from acids, Na2WO4 promotes fluorescence for lanthanides at pH = 4.5. The analysis of UO2 (SO4) within H3PO4 by Tl (I) ions in the range of 0.1-80 ppm is completed along with fluorescence quenching. Unfortunately several of the ions show strong interference and reduce fluorescence intensity. Somemetals and the reagents used for the determination along with the spectral features, sensitivity and the interferences are given in Table.
Table: Fluorimetric determination of inorganic materials