Photoluminescence and Structure:
We will first explain the relationship among the structure and the features of fluorescence of a molecule. There is no exact requirement in a molecule which makes it fluorescent. Thus, there are certain general observations. For example, the presence of the benzene ring and the nature of substituents on it seem to favour the fluorescent behaviour of the molecule. Halogen substituents tend to reduce the fluorescence and shift the fluorescence bands to longer wavelengths; the effects increase along with increase within the atomic mass of the substituted halogen.
Compounds along with fused ring are found to be especially fluorescent, and the extent of fluorescence is found to be straight proportional to the number of rings in the molecule. The structural rigidity within a molecule favours fluorescence. For example, fluorescein is a highly fluorescent molecule, although the structurally same phenolphthalein is fundamentally nonfluorescent.
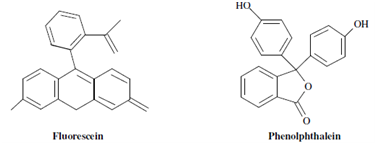
The increase in fluorescence is because of the lack of collisions in the rigid molecules. Another example is biphenyl which has a low fluorescence quantum efficiency that is ~ 0.2 while fluorene with a rigid structure has a quantum efficiency almost equivalent to 1.