Aliphatic and alicyclic carbonyl compounds:
Creation also leads to the rigidity in molecules and displays higher fluorescence. For example, 8-hydroxyquinoline while complexed along with zinc ions increases its fluorescence to a good extent.
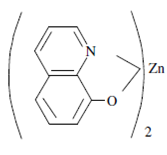
8-Hydroxyquinoline complexed along with zinc ions
The fluorescence observed along with rigid cyclic molecules within π-bonds is found to be enhanced through electron donating groups e.g., -NH2, OR, - OH and -OCH3, although the electron withdrawing groups such as NO2, COOH, N=N and Br, I and CH2COOH tend to reduce it. Instead the nonrigid molecules do not fluoresce much, since these rapidly lose the absorbed energy by nonradiative means such as, vibrational relaxation or even degradation. The substituents on the aromatic ring might affect both, the intensity and the position of the fluorescence band.
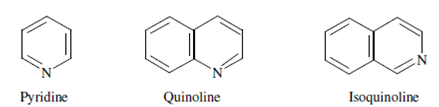
Another observation is that the easy heterocyclic compounds like pyridine, furan, thiophene and pyrrole do not fluoresce significantly, but while fused to a benzene ring, they might become highly fluorescent. For instance, the heterocyclic pyridine is nonfluorescent, but quinoline and isoquinoline in that the pyridine ring is fused to a benzene ring are highly fluorescent.
Aliphatic and alicyclic carbonyl compounds or highly conjugated double bond structures also display fluorescence.