Analysis of Amino Acids and Proteins
Quantification of total protein content within a sample is general to several applications in basic science and clinical research. Over the years, several different absorbance based colorimetric methods to quantify protein have been developed. These methods work well thus, these are subject to interference through several compounds. Various methods based on fluorescence measurements have been developed for protein estimation. Fluorescamine and o-phthalaldehyde have been used along with success to quantify protein content of samples.
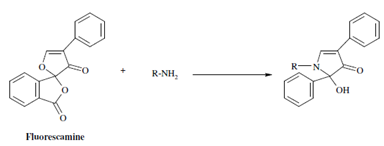
As regards the protein samples, three amino acids constituting them exhibit natural fluorescence. These are tryptophan, tyrosine and phenylalanine; other amino acids thus, require an appropriate agent to convert them to fluorescent derivatives. Fluorescamine, a heterocyclic dione, is extremely useful agent for the analysis of the non fluorescent amino acids. It reacts along with the primary amino group of the amino acid to form a fluorescent product. The fluorescence of a solution holding protein and fluorescamine is found to be proportional to the quantity of free amino groups present. Thus this reaction forms the basis of fluorimetric assay of the proteins and amino acids. The Fluorescamine has also been used in labelling casein-the milk protein, so in which it could be used as a substrate for measuring protease activity.
Another extremely important reagent is o-phthalaldehyde (OPA). It is probably the most hugely used reagents and has sensitivities in the femtomole range. The reagent reacts along with the primary amino group at alkaline pH ranges within the presence of certain thiols such as mercaptoethanol or ethanethiol. A reaction is quite quick and is fundamentally complete within a minute.