Indirect Analysis Methods:
As is obvious from the name, within indirect analysis methods the fluorescence is not measured straight. There are two strategies of indirect methods of analysis. In the first strategy the analyte to be determined is suitably derivatised to a fluorescent species. For instance, 2-hydroxybenzaldehyde (salicyaldehyde) forms bonds along with metals and the resulting species is fluorescent as displays.
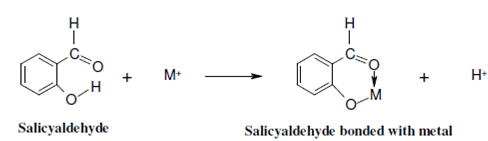
The origin of fluorescence in the molecule might be attributed to the elongation of the chromophore and also to the creation of a ring. The determination of selenium is another instance of indirect determination. In that case diaminonaphthalene is used as a fluorescent label. In indirect methods of analysis it's desirable in which the metal ion to be determined and the ligand and both are nonfluorescent. This facilitates the measurement of the fluorescence of the complex. In that case one of the components is fluorescent; it must have extremely weak fluorescence.
Within the second strategy used in the indirect techniques one has to look for a fluorescent species whose fluorescence is quenched through the analyte. In like a case the fluorescence intensity of the target molecule is measured as a function of the concentration of the analyte. For instance, halide ions quench the fluorescence of quinine. Their concentration could be measured through the quenching method. Another interesting instance is the general fluorescence quencher, O2. This paramagnetic species could also be determined through the quenching method. The quenching method, therefore, has a limitation. It will be meaningful only while the analyte is the only species in which will cause quenching of the fluorescent molecule, but it is little unlikely.