Flame And Its Characteristics:
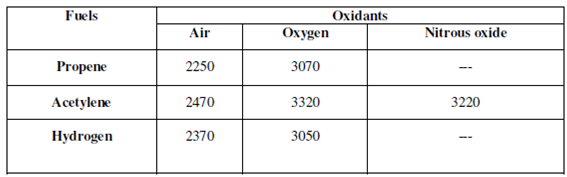
A flame could be described as a steady state gas phase reaction that takes place along with emission of light. It is produced through burning a mixture of fuel and air or oxidant within a burner. Inside flame photometry a variety of fuels could be used and commonly air, oxygen or nitrous oxide (N2O) is used as the oxidant. The flame temperature depends on fuel- oxidant ratio. Combination of a given fuel gas along with air produces lower temperatures as compared to when O2 is used as oxidant. Alkali and alkaline earth metals are easily excited at low temperatures obtained by using air. Therefore, for heavy metals O2 or N2O are employed as oxidants. The requisite temperature for analysis can be acquired through varying the fuel-oxidant ratio. Table lists a few common fuels and oxidants along with range of temperature attainable through using them.
Table: Fuel-oxidant mixtures and the attainable temperature