Various Modes Of Energy
We have met three modes of energy till now as follows:
• Gravitational potential energy
• Kinetic energy
• Internal energy
There are many other modes of energy; some of them are discussed as follows:
Chemical Energy:
Throughout a chemical reaction, reactants are transformed into products; for illustration, whenever petrol vapor and air burn to produce carbon dioxide, water-vapor and other combustion products in a petrol engine. This might be accompanied by heat and work communications, and modifications in properties like temperature and pressure. In specific, when the reaction vessel is rigid and insulated, and hence the energy is constant, the temperature of the products will experience a significant increase in this “exothermic” reaction. In such a condition, we might state that the chemical energy is converted into internal energy.
Strain Energy:
A compressed spring or elastic structure has portion of its energy in the form of strain energy. An associated form of energy is that linked with capillarity or surface tension; systems displaying this phenomenon are thought to have portion of their energy in the form of “surface energy”.
Electrical Energy:
A system including electrically charged elements is thought to have part of its energy in the form of “electrical energy”.
Magnetic Energy:
A system including magnetic poles contains part of its energy in the form of “magnetic energy”.
When both magnetic and electrical effects are concurrently present, their communications also generate a contribution to the total energy of the system.
In summing up, the First Law exemplifies two distinct declarations:
• A system can interact with its surroundings in only two manners, viz., work and heat.
• There is a property termed as energy whose change measures the total effect of such interactions.
Energy has two features:
• It is transformed for and process undergone by an isolated system.
• It is additive that is; it is an ‘extensive’ property.
Example:
The figure shown below illustrates a rough inclined plane with a rectangular block latent on the sloping face. The block is a poor conductor of heat whereas the plane is a good conductor. The block slides gradually down the plane that increases in temperature. State whether the heat, work and rise in energy are positive, negative, or zero, for (a) block, (b) plane, and (c) the block and the plane.
Solution:
Refer to the figure shown below.
In this situation, the block is a poor conductor of heat (like wood), whereas the plane is a good conductor (like copper). Note that as the block slides downward the plane, the latter increase in temperature.
The answer is contained in the table shown below:
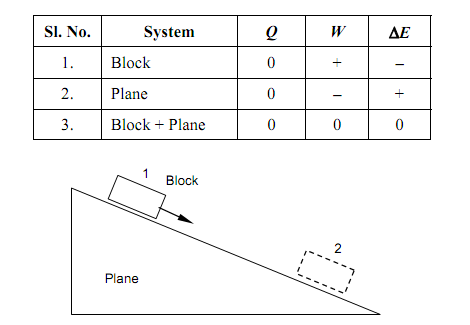
Figure: Block sliding down a rough inclined plane
Explanation:
Block
• Q is zero since the system is a poor conductor of heat; no heat transfer can take place at its interface with the plane even however the latter is at a higher temperature.
• W is positive since, even however the real external effect is a rise in temperature of the plane, the block could have carried out the similar process, and had as its solitary effect the increasing of a weight; for illustration, when the plane had been lubricated and the block associated to an appropriate pulley mechanism.
• As (Q – W) is therefore negative, it obeys that ΔE is negative. The block experiences a reduction in its gravitational potential energy. Plane
• Q is zero for the same purpose it is zero for the block; no thermal interactions.
• W is negative for the plane, since it is positive for the block.
• Therefore (Q – W) is positive, and therefore is ΔE. It is the internal energy of the plane that has risen.
Block + Plane
The joint system, block + plane, does not interact with its surroundings in any way. Therefore, both Q & W are zero for this system. By definition, thus, ΔE also equivalents to zero. The reduction in the gravitational potential energy of one portion of the system (i.e., the block) is precisely balanced by the raised internal energy of the other part of the system (i.e., the plane).