Statement Of The First Law For Non-Cycic Processes
The definition of the First Law for a non-cyclic process is as shown below:
Q – W = ΔE
Here the symbol ΔE symbolizes the rise in the magnitude of energy E throughout the process, that is, its final value minus its original value. It entails that E is a property. (i.e., Δ cannot be exerted to Q or W that are not properties).
Example:
With reference to the figure shown below, whenever a system is taken from A to B all along ACB, 100 kJ of heat is transferred to the system that executes 30 kJ of work.
(a) Determine the heat transfer to the system all along the path ADB when the work done is 10 kJ.
(b) If the system is returned from B to A all along the curved path, 20 kJ of work is completed on the system. Compute the magnitude and sign of the equivalent heat transfer.
(c) When EA = 0 and ED = 40 kJ, compute the heat transfers during the procedures AD and DB.
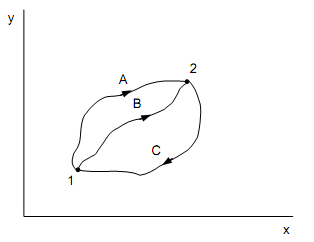
Solution:
Refer to the figure shown above,
For the path ACB, according to the First Law,
Q – W = ΔE; 100 – 30 = ΔE
Therefore, EB – EA = 70 kJ
(a) For the path ADB, according to the First Law,
Q – 10 = EB – EA = 70
Therefore, Q = 80 kJ
(b) For the curved path BA, according to the First Law,
Q – (– 20) = EA – EB = – 70
Therefore, Q = – 90 kJ
(c) EA = 0; ED = 40 kJ
For the procedure AD, ΔE = ED – EA = 40 kJ
W = work done throughout ADB, as the work throughout the constant volume procedure DB is zero
= 10 kJ.
From the First Law, Q – 10 = 40,
And hence, Q = 50 kJ.
For the procedure DB, W = 0 (i.e., Constant volume process)
ΔE = EB – ED = (EB – EA) – (ED – EA)
= 70 – 40 = 30 kJ
Form the First Law, Q – 0 = 30 kJ, and hence Q = 30 kJ.