Statement Of The First Law For Cyclic Processes
The outcomes of James Prescott Joule classes of experiments might be generalized to the following statement of the First Law:
“Whenever a system executes a cyclic process, the total work is proportional the total heat”.
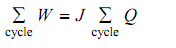
The above equation is applicable to procedures in which the heat and work interactions take place in finite steps. Whenever the interactions take place as a succession of infinite steps, the summation signs are substituted by integrals, resulting the symbolic form of the law as shown:
∫ δ W = J ∫ δ Q
Here δQ and δW symbolize minute elements of the heat and work communications, and stands for the “cyclic integral” or “integral about the cycle”.