Experiments Utilizing Heat and Work to Obtain Equivalent Effects
The figure (a) shown below illustrates a procedure during which 1 kg of water is increased in temperature by a specified amount as an outcome of thermal communication with a hot body at a higher temperature. An accurately similar change in the water can be completed without any heat transfer at all; the figure (b) shown below illustrates the water being stirred by a paddle wheel within a thermally insulated vessel, therefore increasing its temperature.
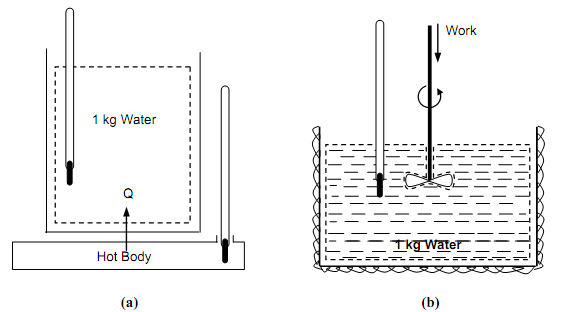
Figure: Experiments in which the similar effect is attained (a) By Heat and; (b) By Work
Therefore, heat and work can bring about equal effects. Joule carried out most of the experiments of this kind with different systems and various kinds of work. He established that, inside the limits of exactness of his experiments, the number of units of work needed to establish a specified effect was proportional to the number of units of heat needed to bring about the similar effect. Symbolically, it meant that W/Q = a constant, here W and Q are the work and heat interactions that cause similar change of state in alike systems. The constant was termed to as the “Mechanical Equivalent of Heat”, was specified by the symbol J, with a value of 4.1868 × 103 N.m/kcal; in SI units, and J = 1 newton metre per joule.