First Law Of Thermodynamics
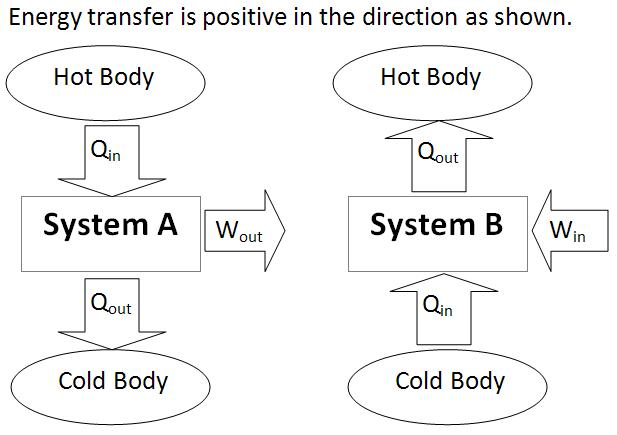
The first law of thermodynamics is a description of the law of conservation of energy, specific for thermodynamically systems. It is generally formulated by defining that the change in the internal energy of a closed system is equivalent to the amount of heat supplied to the system, minus the amount of work done by the system to its surroundings. The law of the conservation of energy can be defined: The energy of an isolated system is constant.
The first explicit definition of the first law of thermodynamics, by Rudolf Clausius in the year1850, referred to cyclic thermodynamic procedures.
"In all situations, in which work is generated by the agency of heat, an amount of heat is consumed that is proportional to the work done; and on the contrary, by the expenses of an equivalent quantity of work an equivalent quantity of heat is generated."
Clausius defined the law also in another form, this time referred to the existence of a function of state of the system termed as the internal energy, and stating himself in terms of a differential equation for the increments of a thermodynamic procedure. This equation might be translated into words as follows:
In a thermodynamic procedure of a closed system, the increase in the internal energy is equivalent to the difference among the increment of heat build up by the system and the augmentation of work done by it.
The first law of thermodynamics was stated in two ways by Clausius. One way referred to the cyclic processes and the other way referred to any incremental change in the internal state of the system.