Control Volume Concepts
The understanding of control volume notion is necessary in analyzing a thermodynamic problem or building an energy balance. The two basic approaches exist in studying Thermodynamics: the control mass come near and control the volume approach. The previous is termed to as the LeGrange approach and the later as the Eulerian approach. In the control mass notion, a "clump" of fluid is study with its related energies. The analyzer "rides" with the clump where it goes, keep balances of all the energies disturbing the clump.
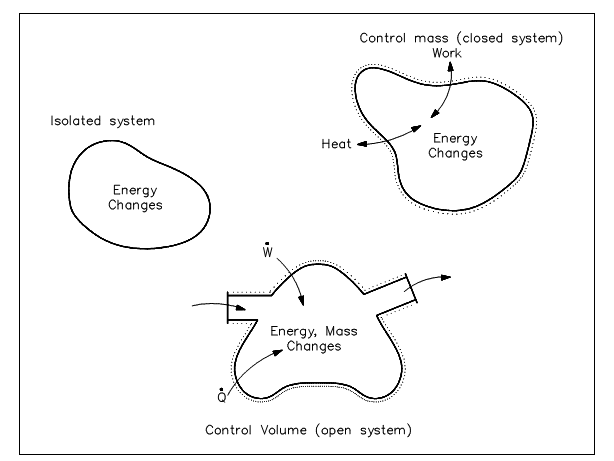
Figure: Control Volume Concepts
The control volume approach is one in which a fixed area in space is recognized with identified control boundaries, as shown in figure above. The energies which cross the boundary of this control volume, involving those with the mass crossing the boundary, are then deliberate and the balance executed. The control volume approach is generally used nowadays in analyzing thermodynamic systems. It is more suitable and needs much less work in maintaining track of the energy balances. Illustrations of control volume applications are involved in the figures shown below.
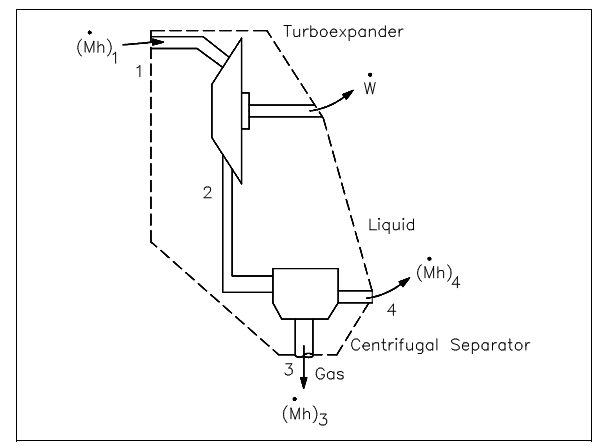
Figure: Open System Control Volumes
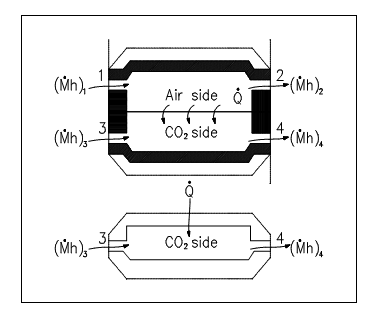
Figure: Open System Control Volumes (Cont.)
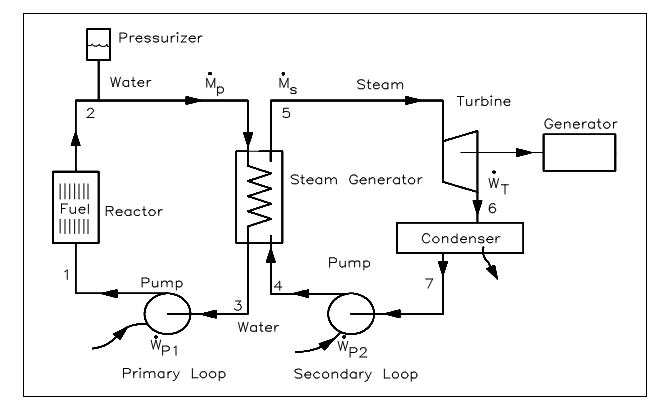
Figure: Multiple Control Volumes in similar System
The kinds of energy which might cross the control volume boundary involve those related with the mass (m) crossing the boundary. The Mass in motion has potential (PE), kinetic (KE), & internal energy (U). Additionally, as the flow is generally supplied with some driving power (i.e., a pump for illustration), there is other form of energy related with the fluid caused by its pressure. This kind of energy is termed to as flow energy (Pν-work). The thermodynamic words therefore presenting the different forms of energy crossing the control boundary with the mass are specified as m (u + Pν + ke + pe).
In open system study, the u and Pν words take place so often that another property, enthalpy, has been stated as h =u + Pν. This outcomes in the over expression being written as m (h + ke + pe). In adding up to the mass and its energies, outwardly exerted work (W), generally designated as shaft work, is the other form of energy which might cross the system boundary.
In order to complete and please the conservation of energy association, energy which is caused by neither mass nor shaft work is categorized as heat energy (Q). Then we can explain the association in equation form as follows.
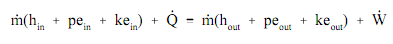
Here:
m = mass flow rate of working fluid (lbm/hr).
hin = specific enthalpy of the working fluid entering the system (Btu/lbm)
hout = specific enthalpy of the working fluid leaving the system (Btu/lbm)
pein = specific potential energy of working fluid entering the system (ft-lbf/lbm)
peout = specific potential energy of working fluid leaving the system (ft-lbf/lbm)
kein = specific kinetic energy of working fluid entering the system (ft-lbf/lbm)
keout = specific kinetic energy of working fluid leaving the system (ft-lbf/lbm)
W = rate of work done by the system (ft-lbf/hr).
Q = heat rate into the system (Btu/hr).
It exhibits that potential and kinetic energy words are unimportant for a turbine, as the ?pe and ?ke values are below than 1 Btu/lbm.