pH:
When we say pH of the aqueous phase, it essentially means the equilibrium pH i.e., the pH attained after the two phases have been contacted to equilibrium. This is a dominant variable where H+ ion is involved in the formation of the extracting species. This will mean that pH will be of great significance in extraction systems listed in the classification scheme under "Extraction by compound formation". The extraction through chelating agents, carboxylic and sulphonic acids and acidic organophosphorus compounds are susceptible to pH variation. For illustrate the point, the extraction behaviour of some metal ions through dithizone, Versatic 9. and DEHPA is shown in Figures respectively. Since expected, the extraction increases with the increasing pH.
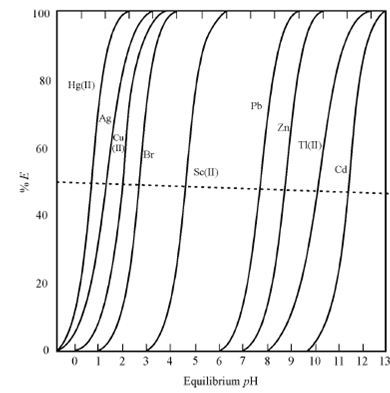
Figure(a): Qualitative extraction curves for metal dithizonates
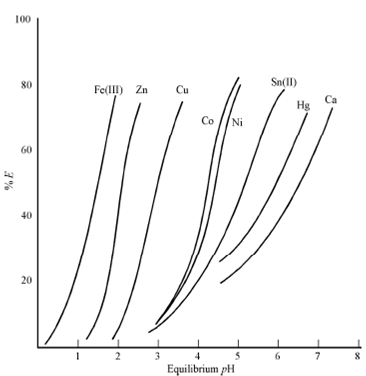
Figure(b): Extraction of Fe, Cu, Zn, Ni and Co with Versatic 9