Molarity of the Acid:
When we say the molarity of an acid, it invariably implies the range of acidity which is not going to be covered through the pH scale. A study of the effect of molarity of the acid on extraction is carried out on the extraction of metals by solvation or ion - pair formation. These investigations are mainly confined to the mineral acids and that too mostly to HCl and HNO3.
Figures depict the extraction behaviour in the two types of extraction systems. Below figure gives the extraction behaviour of 3d transition metal ions viz. Ti (IV), V (IV), Cr (III), Fe(III), Mn(II) Co(II), Ni(II), and Cu(II) from HCl solution in a toluene solution of Cyanex 923. An extraction profile of a few commonly associated elements like Ce(IV), Al(III), Ga(III), Mg(II), Cd(II) and Pb(II) commonly encountered in some of the matrices is also given.
The dependence of percent extraction from 1- 10 M HCl is shown. The extraction of Zn(II) and Cd (II) is more or less quantitative (>95%) over the entire acidity range. The extraction of Ti (IV), V (IV), Fe (III), Co (II), Ga(III) and Pb (II) increases with the increasing acidity. The extraction of Cu (II) shows a maxima around 5 M HCl. Mn(II) shows a negligible extraction (< 5%) upto 5 M HCl; thereafter, it increases to a maximum of 30% at 10 M HCl. The extraction of Mn is not shown in the figure.
The extraction of Ce (IV), Al (III), Cr (III), Mg (II) and Ni (II) is negligible over the entire acidity range and is not shown in the figure. The extraction observed here is governed by a complex mechanism promoting the formation of solvated neutral chloro species. Sometimes, the mutual solubility of the two phases plays a significant role. The maxima are observed due to competing factors coming into play to determine the magnitude of extraction.
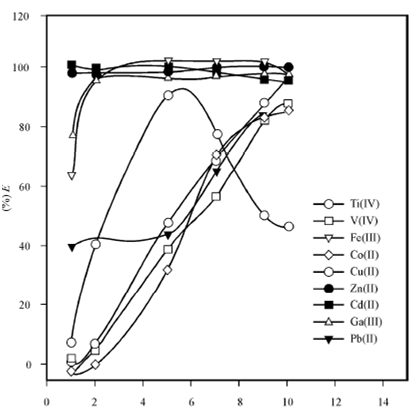
Figure: Extraction behaviour of metal ions from HCl medium. Conditions: [Metal ion] = 1x10-3M; [Cyanex 923] = 0.5 M