Effect of Metal Ion Concentration:
It has been emphasized in the beginning that there is no effect of the metal ion concentration on the distribution ratio of the metal. This will mean that both tracer and macro amounts of metals may be expected to extract to the same extent under similar equilibrium conditions provided the solubility of the extracting species in the organic phase is not exceeded. The relationship among Maq. and Morg. With the increasing metal ion concentration is used to find the loading capacity of the extractant. These plots known as loading curves or extraction isotherms for the extraction of Ti (IV), V (IV), Fe (III), Cu (II) and Zn (II) in toluene solution of Cyanex 923 are shown in Figure.
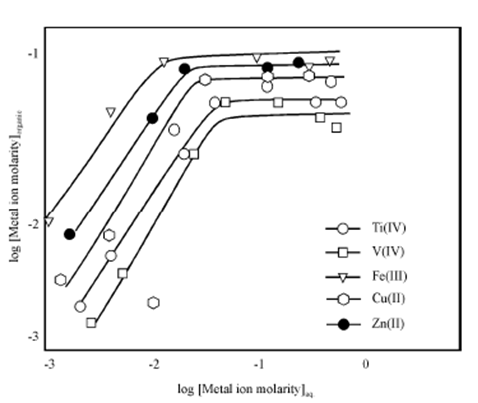
Figure: Effect of metal ion concentration on extracti on of Ti(IV), V(IV) Fe(III), Cu (II) and Zn (II). Conditions : [Cynex 923] = 0.2 M; [HCl] = 5M
In all these plots, the linear part of the curve means that the extracting species does not change with the increasing metal ion concentrations, thereafter, at a certain point, the loading condition sets in and no further increase in the metal content of the organic phase is observed. From this, the amount of the metal ion that can be loaded on a particular amount of the extractant can be calculated and the results expressed in terms of molar ratio. Sometimes from this, you can infer the stoichiometry of the extracted species.