Alkyl halides -SN1:
Steric and electronic factors as well play a vital role in the rate of the SN1 reaction. Because the steric bulk of three alkyl substituents create it extremely difficult for a nucleophile to arrives at the electrophilic carbon center of tertiary alkyl halides, these structures go through nucleophilic substitution by the SN1 mechanism instead. In this mechanism, the steric problem is relieved as loss of the halide ion makes a planar carbocation in which the alkyl groups are much further apart and in which the carbon center is more accessible. Creation of the carbocation also relieves steric strain among the substituents.
Electronic factors also assist in the creation of the carbocation because the positive charge can be stabilized through the inductive and hyperconjugative effects of the 3 alkyl groups.
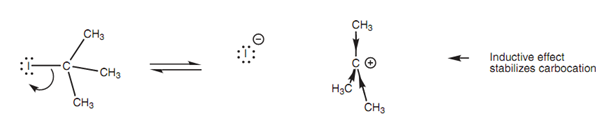
Figure: Inductive effects stabilizing a carbocation.