Alkyl halides - SN2:
There are two issues that affect the rate at which alkyl halides go through the SN2 reaction - electronic and steric. In order to demonstrate why different alkyl halides react at diverse rates in the SN2 reaction, we shall compare a primary, secondary, and tertiary alkyl halide as shown in figure.
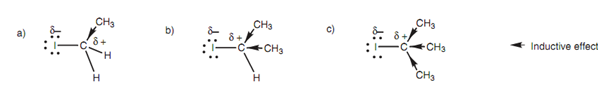
Figure: (a) Iodoethane; (b) 2-iodopropane; (c) 2-iodo-2-methylpropane.
Alkyl groups comprise an inductive, electron-donating effect that tends to lower the electrophilicity of the neighboring carbon center. The meaning of lowering the electrophilic strength is that the reaction center will be less reactive to nucleophiles. Hence, tertiary alkyl halides will be much less likely to react with nucleophiles as compared to primary alkyl halides, because the inductive effect of three alkyl groups is greater than one alkyl group.
Steric factors as well play a role in making the SN2 mechanism hard for tertiary halides. An alkyl group is a bulky group as compared to a hydrogen atom, and hence can act like a shield against any incoming nucleophile described in figure. A tertiary alkyl halide comprises 3 alkyl shields as compared to the one alkyl shield of a primary alkyl halide. Hence, a nucleophile is more likely to be deflected while it approaches a tertiary alkyl halide and fails to reach the electrophilic center.
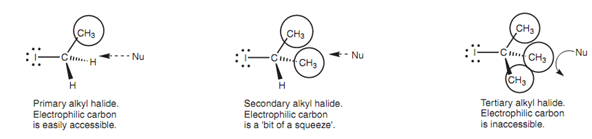
Figure: Steric factors affecting nucleophilic substitution.