Extraction equilibria:
In Eq. for the extraction equilibria, the concentration of the metal ion does not figure. This means that the extraction is independent of the metal ion concentration. In other words, it implies that whether it is tracer level concentration or macro amounts extraction would be the same.
At this point, it may be important to consider the role of [H+] or pH and for this, we again resort to Eq.
D= KfKnaKDX/KnDR [HR]no/[H+]na
If KfKnaKDX/KnDR = K*
D= K* [HR]no/[H+]na
Taking log of both sides,
log D = log K* + n log [HR]o -nlog[H+]a
log D = log K*+ n log[HR]o + n pH
Therefore, it means that plot of log D vs. pH should be a straight line with slope n and an intercept equal to (log K* + n log [HR]o). Figure shows such a plot. The slope n gives the number of molecules of the chelating agent involved in neutralizing the charge on the metal ion. Through plotting the data at various pH, the values of both n and K* can be found out.
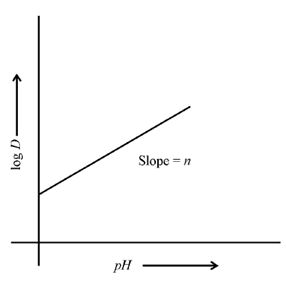
Figure: A typical plot between pH and log D