Dithizone:
The other well known chelating agent used for solvent extraction is diphenyl thiocarbazone known by the popular name "dithizone".
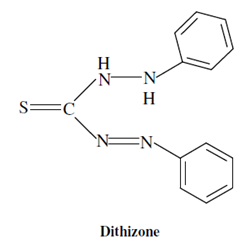
Dithizone and its complexes with metal ions are insoluble in water but soluble in chloroform or carbon tetrachloride. A list of metals creating chelates includes Mn, Fe, Co, Ni, Cu, Zn, Ag, Cd, Sn and Pb.
Let us consider that a metal ion with a charge 'n' reacts with an extracting chelating reagent to form the metal chelate. While the aqueous phase containing the ligand (HR) is brought in contact along with the organic phase, a reagent distributes itself within both the phases till the equilibrium is attained.
(HR)a ↔ (HR)o
The distribution coefficient of the ligand, KDR, is given via
KDR = [HR ]o /[HR ]a
Further, the ligand dissociates in the aqueous phase as follows:
HR ↔ H+ + R-
The dissociation constant of the ligand, Ka , is expressed as
Ka = [H + ] a [R - ]a /[HR ]a