Dependence of distribution ratio:
Not only this, the extraction of anionic organic complexes like oxalate, citrate, malonate takes place by similar mechanism. It is clear from the above examples that the transfer of an ion pair to the organic phase is by a mechanism similar to uptake by a solid anion exchanger. This is why the high molecular weight amines are popularly known as liquid anion exchangers. Generally, the pattern of the extraction behaviour is similar to the uptake of the metal ion on the solid anion exchange resin. One feature which is clear from the above equilibria steps is that the extraction (D) of the metal ion will increase with the increase in amine concentration. A plot of log D vs log [Amine] should be linear (see Fig.3.2) and the slope of this straight line will give the number of amine molecules involved in the formation of the extracting species. This will give the charge on the anionic metal complex and the extracting species can be postulated.
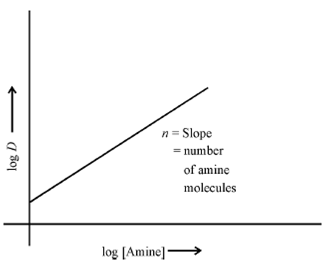
Figure: Dependence of distribution ratio of the metal ion on the amine concentration at constant aqueous phase conditions.