Extractants Containing Phosphorus-Oxygen Bonds:
Extractants of this group could be considered as derivatives of phosphoric acid. The general structures of the esters of organophosphorus acids are given in Table.
Table: General Structure and Examples of Esters of Organophosphorus Acids
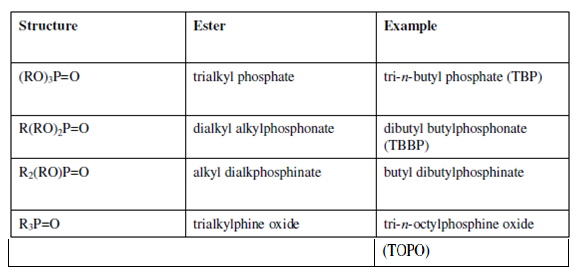
The first three members of this group are esters of phosphoric, phosphonic and phosphinic acids and the last member is a derivative of phosphine. The extraction mechanism for all of them is mainly the same. It is the oxygen of the phosphoryl group which is responsible for the coordination bond formed with the metal. In the case of esters, there is a possibility of formation of more than one coordination bond through other oxygen atoms. The inter or intramolecular bifunctional complex, thus, formed exhibits different extraction and stripping rates. The solubility of these extractants in water is very much in the predictable order: phosphates < phosphonates < posphinates < phosphine oxide. This sequence is in line with the increasing polarity of the phosphoryl group in the molecule. The solubility decreases with the increasing chain length.