Dissociation of the ion pair complex and the reagent in the organic phase:
At extremely low Fe (III) concentrations, the dissociation of extractable complex and the reagent occurs in the ether phase.
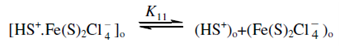

Now assuming the overall distribution of iron in its different forms between the organic and the aqueous phases.
D ≡ [Fe]o /[Fe]a
= [(HS+.FeCl-4]o +[Fe(S)2Cl-4]o +n[(HS+.Fe(S)2Cl-4)n]o/ [FeS2Cl-4]a +[(HS+.Fe(S)2Cl-4)]a
It can be seen that the distribution ratio is a complex function of different experimental parameters and by substituting the relations form previous equations, we get
D= K'D K7[HS+][1+K11{KDK7K11[HS+][Fe]+K12KD[HS+][Cl-]-1/2 +. nK10(KDK7)n-1 [HS+]n-1[Fe]n-1]
In the above Equation i.e., Eq.3.32 the two important variables are acidity and the total iron concentration. By this equation, the following inferences can be drawn:
i) In relatively high iron concentration where polymerization takes place to a significant extent and dissociation is relatively unimportant, Eq.simplifies as follows:
D ≅ K D′ K7[HS+ ] {1+nK10 (KD K7)n-1[HS]n-1[Fe]n-1}