Carboxylic and Sulphonic Acids:
The solutions of carboxylic and sulphonic acids, in non-polar organic solvents, are similar to cross linked resinous cation exchangers. They frequently offer some advantages and often only require a pH adjustment of the aqueous phase for achieving the separation of elements similar in properties. Different carboxylic, alkyl or aryl-alkyl sulphonic and sulphuric acids form salts with a number of metal ions including alkali and alkaline earths. These salts are frequently less soluble in aqueous solutions but more in some organic solvents. The extractability of these acids generally parallels their basicity.
The metal extraction by these extractants is complicated by their state of aggregation in solution. The polymerization of these acids, although, does not go beyond dimers but the extensive aggregation of their salts plays a significant role in their extraction. The bulk of available data on the extraction by carboxylic acids is on normal or branched chain mono carboxylic acids with seven or more carbon atoms in the chain. Under certain specified conditions, the aliphatic and aromatic hydroxyl carboxylic and dicarboxylic acids can also extract certain metal ions. Various benzoic acid derivatives, dinitrobenzoic, cinnamic and phenylacetic acids and amino and perfluoro derivatives of n-carboxylic acids have also been used as extractants. Out of commercial extractants of this class, Versatic and Naphthenic acids obtained from the distillation of crude petroleum are important.
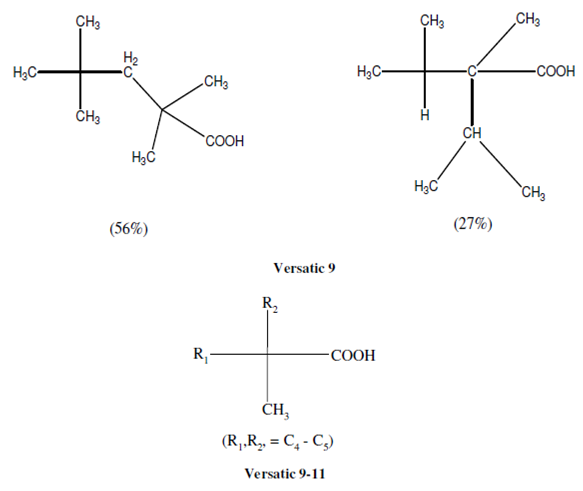
Other Versatic acids having a similar structure are Versatic 10, 13 and SRS-100.