Inductive effects:
Stabilizing the conjugate base's negative charge is significant in determining the strength of the acid and thus any influence that stabilizes the charge will result in a stronger acid. Substituents can assist to stabilize a negative charge and do so through an inductive effect. This is demonstrated by comparing the pKa values of the alcohols CF3CH2OH and CH3CH2OH (12.4 and 16, correspondingly) in which CF3CH2OH is more acidic than CH3CH2OH. This implies that the anion CF3CH2O is more stable as compared to the CH3CH2O .
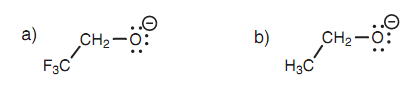
Figure (a) 2 ,2, 2-Trifluoroethoxy ion; (b) ethoxy ion.