Ring opening of an epoxide:
Ring opening within basic conditions is as well possible with heating, but needs the loss of a negatively charged oxygen described in figure. This is a poor leaving group and would not take place along with normal ethers. It is only possible here as the reaction opens up the epoxide ring and relieves ring strain.
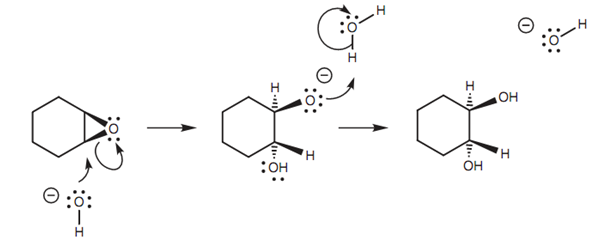
Figure: Mechanism for the ring opening of an epoxide under basic conditions.
Ring opening through the SN2 reaction is as well possible by using nucleophiles other than water. Through unsymmetrical epoxides, the SN2 reaction will take place at the least sub- stituted position if it is performed within basic conditions.
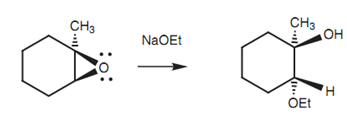
Figure: Ring opening of an epoxide with the ethoxide ion.
Though, under acidic conditions, the nucleophile will generally attack the most substituted position. This is since the positive charge in the protonated intermediate is shared among the oxygen and the most substituted carbon. This makes the more substituted carbon much more reactive to nucleophiles.